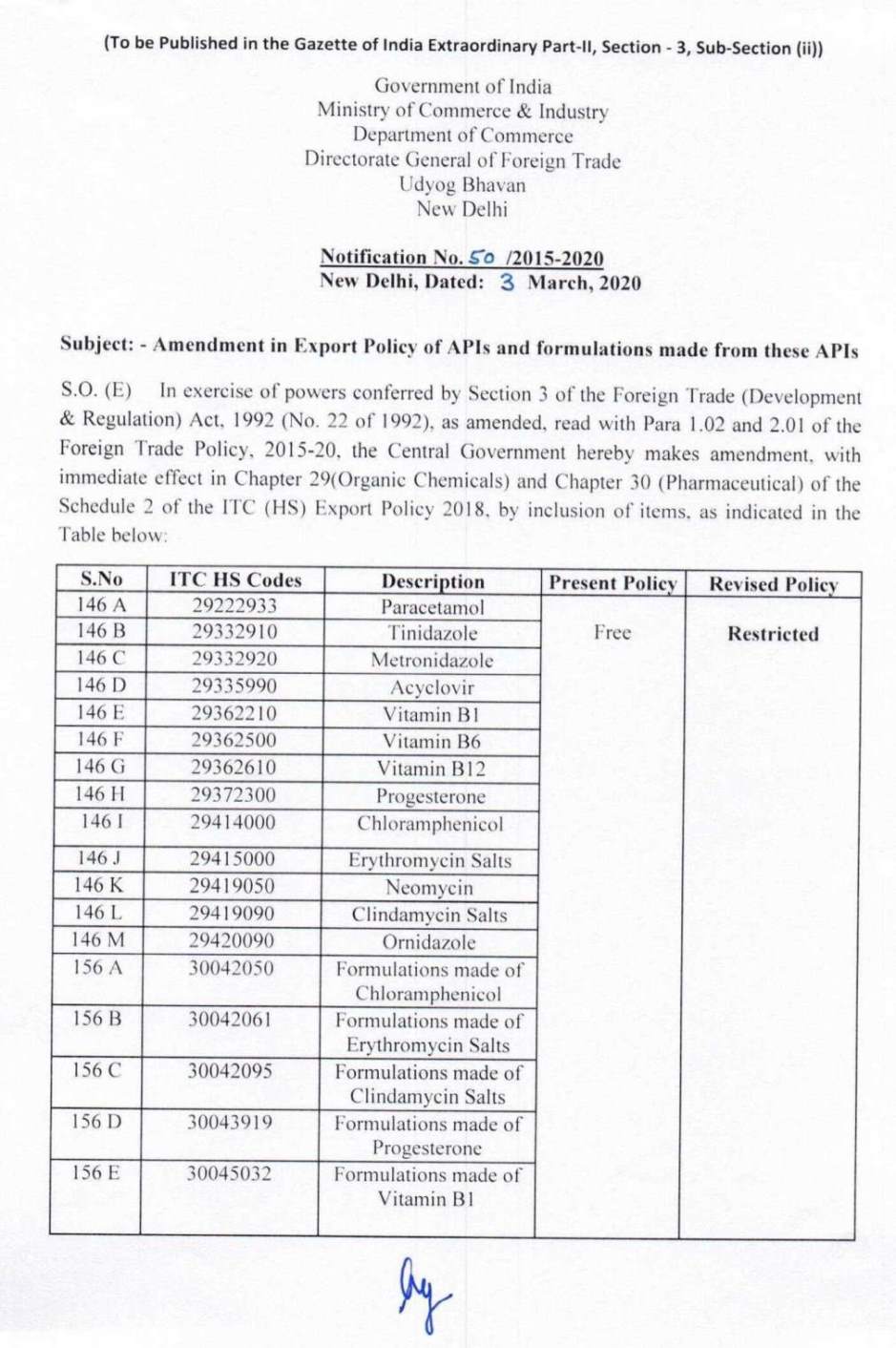
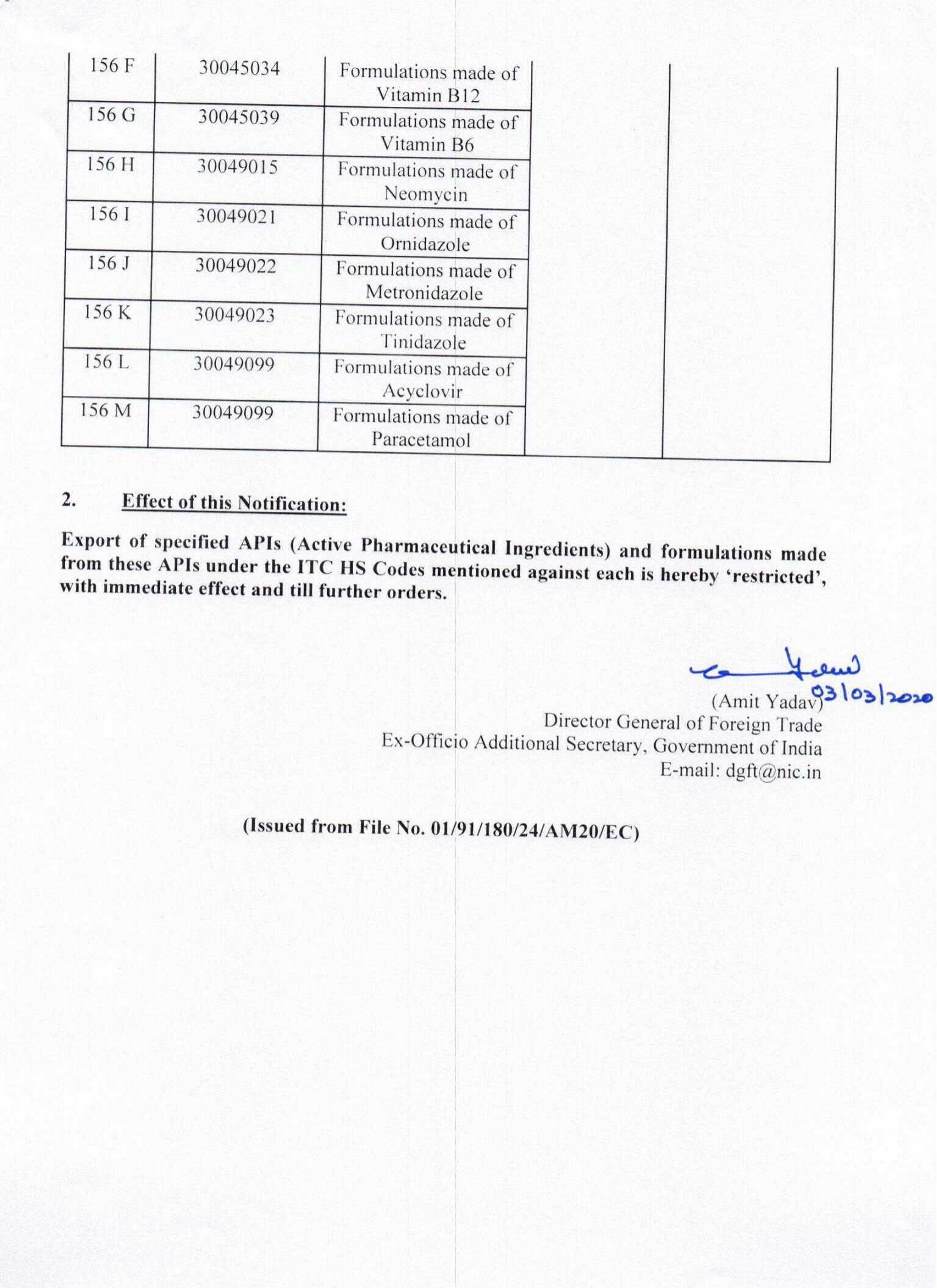
S.O.(E) In exercise of powers conferred by Section 3 of the Foreign Trade( Development & Regulations) Act, 1992(No.22 of 1992), as amended, read with Para 1.02 and 2.01 of the Foreign Trade Policy, 2015-20, the Central Government hereby makes amendments, with immediate effect in Chapter 29(Organic Chemicals) and Chapter 30(Pharmaceutical) of the Schedule 2 of the ITC (HS) Export Policy, 2018, by inclusion of items, as indicated in the notification: (Notification No. 50 /2015-2020)
All medical devices to be treated as ‘drugs’ from April 1: Govt of India The central government on Tuesday notified all medical devices as ‘drugs’, effective from April 1, bringing a range of products from medical use in human beings or animals under Drugs Act, 1940. Apart from expanding the scope of regulation to ensure safety and efficacy, the move may pave the way for regulation of prices under the Drugs Price Control Order (DPCO). It will also make companies, in case of […]